 Heard a very good Renal Grand Rounds yesterday from visiting professor Michael Emmett, currently chair of the Dept. of Medicine at Baylor, regarding the anion gap.
Heard a very good Renal Grand Rounds yesterday from visiting professor Michael Emmett, currently chair of the Dept. of Medicine at Baylor, regarding the anion gap.
Amongst the pearls I took away from his lecture:
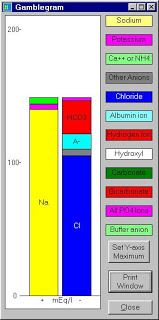
1. “Gamblegrams” (left), initially created by acid-base pioneer James L. Gamble, are a useful way of analyzing most acid-base disturbances and helping to visualize the anion gap.
2. In the U.S., the anion gap is [Na] – ([Cl] + [HCO3]) and the normal range is 8-12; in many other countries (e.g., Europe, Australia) the anion gap is calculated as ([Na] + [K]) – ([Cl]+[HCO3]). The addition of the cation [K] in the equation means that these anion gaps would be slightly lower than those in the U.S. Keep this in mind when interpreting literature on the anion gap from other countries.
3. the main difference between an anion-gap metabolic acidosis (AGMA) caused by organic acids (e.g., lactic acidosis, ketoacidosis) and inorganic acids (e.g., hydrochloric acid) is that organic acids are metabolizable to bicarbonate (resulting in an eventual reversibility to the acid-base disturbance) whereas inorganic acids are not, relying solely on renal bicarbonate synthesis to restore homeostasis.
4. One weird (and not terribly common any longer) cause of acidosis: pseudohyperchloremia bromism. Several decades ago, bromide was a common over-the-counter sedative. Because the laboratory test which detects chloride has an even greater sensitivity for bromide ions, individual with bromide toxicity can actually present with a NEGATIVE ANION GAP! Bromides are less available now, with the exception of the acetylcholinesterase medication Mestinon bromide (pyridostigmine), which is still used to treat myesthenia gravis.


AG will be higher if K is included in the equation (by +4) 🙂