 Magnesium (Mg++) is a predominantly intracellular divalent cation which is critical for many metabolic processes and participates in >300 enzymatic reactions. It is essential for many critical transporters including the Sodium/Potassium ATPase cotransporter. Mg++ also has a critical role for DNA replication, transcription and translation.
Magnesium (Mg++) is a predominantly intracellular divalent cation which is critical for many metabolic processes and participates in >300 enzymatic reactions. It is essential for many critical transporters including the Sodium/Potassium ATPase cotransporter. Mg++ also has a critical role for DNA replication, transcription and translation.
Mg++ intracellular homeostasis is complex because it is compartmentalized in different organelles in the cell with different concentration.
The distribution of Mg++ in the human body is interesting.
- Serum 0.3%
- RBCs 0.5%
- Soft tissue 19.3%
- Muscle 27%
- Bone 52.9%
Therefore measuring serum Mg++ represents an inaccurate way to assess total body Mg++ stores and can be misleading. In addition, there is no standardized normal range for Mg++ and values vary from lab to lab. E.g., at the Brigham & Women’s (1.8-2.5mg/dl), Mass General (1.4-2.0mg/dl) and VA (1.8-2.4 mg/dl) hospitals there is quite a discrepancy in what is considered a normal range.
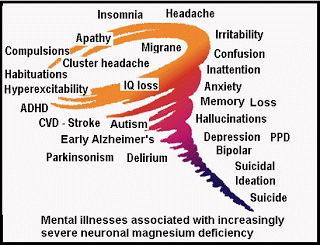
Clinical signs of hypomagnesemia (from mild to severe) include loss of appetite, nausea/vomitting, fatigue, weakness, numbness, tingling, muscle contractions/cramps, seizures, nystagmus, personality changes, hypokalemia, hypocalcemia, arrhythmias and coronary spasms.
Causes of hypomagnesmia can be reduced intake and/or absorption e.g. in malabsorption syndromes, change in redistribution e.g. during exchange transfusions and last but not least reduced renal re-absorption which is the most likely etiology. Causes include alcoholism, diabetes mellitus, hyperthyroidism, hypercalcemia and several medications (loop diuretics, aminoglycosides, cisplatin, calcineurin inhibitors, etc.).
Approximately 75-80% of serum Mg++ is filtered in the glomerulus. 15-20% are re-absorbed in the PT, ~65-75% are re-absorbed in the TAL and 5-10% are re-absorbed in the DCT. The DCT is the site of fine regulation of Mg++ excretion. The fractional excretion of Mg++ can range from 0.5% to 80% but typically is in the range of 3-5%.
How Mg++ re-absorption is regulated in the kidney is still unclear. Compared to other electrolytes there was no hormonal or regulatory known until recently. Its re-absorption appeared to be coupled to Calcium re-absorption. However, rare inherited Mendelian forms of Mg++ disorders have given insight into the molecular mechanisms of abnormal Mg+ homestasis:
Condition: Gene Mode of inheritance tubular location Urine Ca excretion
FHHNC: Claudin16 AR TAL High
BARTTER’s: CLCNKB AR TAL High
ADH: CaSR AD TAL&DCT High
FHH: CaSR AD TAL&DCT Low
NSHPT: CaSR AR TAL&DCT Low
GS: NCCT AR DCT Low
HSH: TRPM6 AR DCT Low
IDH: NaK-ATPase AD DCT Low
Mitoch.: tRNAile Maternal lineage DCT Low
Abbreviations:
AR = Autosomal recessive
AD = Autosomal dominant
ADH = Autosomal dominant hypocalcemia
FHH = Familial Hypercalcemia with hypocalciuria
NSHPT = Neonatal severe hyperparathyroidism
GS = Gitelman syndrome
HSH = Hypomagnesemia with secondary hypocalcemia
IDH = Isolated dominant hypomagnesemia
CaSR = Calcium-sensing receptor
NNCT = Sodium-Chloride Cotransporter
TRPM6 = transient receptor potenetial cation channel, subfamily M, member 6.
A few comments on these inherited diseases which may be relevant. FHH and NSHPT feature typically elevated serum Mg++ levels. Disorders of hypomagnesemia located to the DCT have typically low urine calcium excretion compared to disorders located to TAL which have hypercalciuria. Another difference based on location of defect in the tubulus is degree of “hypermagnesuria”. TAL defects result typically in higher FeMg++ of 15-50%, whereas hypomagnesemia caused by defects in the DCT results in lower levels of FeMg++ (5-15%). Patients with mutation in the gene Claudin 19, a paracellular tight junction protein, have similar phenotype to FHHNC caused by Claudin 16 (Paracellin). It turns out that Claudin 16 and Claudin 19 form a channel like structure to transport Mg++ through paracellular pathway in the TAL. This may be the most important mechanism of Mg++ transport in the TAL.
An interesting new chapter on Mg++ homeostasis began in 2007 based on a report by Bindel and colleagues in JCI. They reported that the EGF receptor may regulate TRPM6, a Mg++ channel in the DCT and that EGF is a magnesiotropic hormone. They reached that conclusion by reporting a family with a recessive form of hypomagnesemia with a loss-of-function mutation in Pro-EGF, a precursor of EGF, leading to lower EGF levels. Further evidence for this mechanism was shown in patients treated with Cetuximab, an anti-EGF-receptor antibody which causes hypomagnesemia.
In summary, Mg++ deficiency is probably more prevalent than recognized since serum Mg++ may or may not reflect intracellular Mg++ stores. Hypomagnesemia is linked to human disease since patients with low serum Mg++ have poorer outcome. Mg++ homeostasis is still poorly understood, however inherited forms of hypo- and hypermagnesemia provide the best understanding of its regulation.
