Welcome to the third case of the Skeleton Key Group, a team of twenty odd nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases that intrigued the treating fellow.
Check out Sayna Norouzi’s video here.
There’s more! A #tweetorial here by Sayna Norouzi.
A. The Stem
A 34 year-old male with a history of asthma and marijuana use presented with nausea and vomiting. Patient was vomiting for 4 days, with approximately 5 episodes per day. No hematemesis. The patient has not been able to keep any food down for the past two days. His mental status has been altered for three days. He was admitted for abdominal pain presumed to be due to retching without diarrhea. His last marijuana use was two days prior to admission. He typically uses every 2-3 days.
Vitals on arrival to the ED: 131/87 mmHg, HR 142 bpm, RR 20, T 98.2, O2 sat 97%. Weight 58 kg.
On exam, he appeared fatigued, AOx3, answering questions but lethargic. He was able to mention he was in a hospital, but not the name and was unable to name the day of the week, but able to answer year. His oropharynx was clear, mucous membranes were moist, there was normal skin turgor, and no lower extremity edema was present.
In the Emergency Department, he was given one liter of Plasmalyte/
B. The Labs
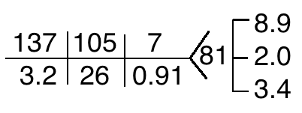
Initial labs (prior to fluid administration):

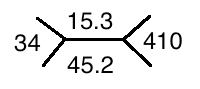
Labs from three months prior to admission:
C. Differential Diagnosis
What do you do next? What is your thought process?
Rule out life threatening situations.
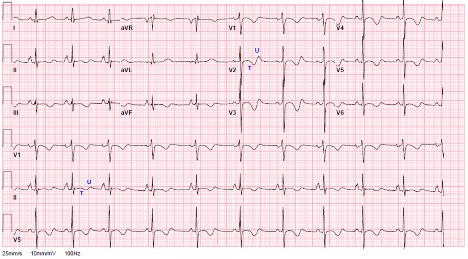
There are many EKG changes seen with hypokalemia.
- Decreased T wave amplitude
- ST segment depression
- T wave inversions
- May have PR interval prolongation with increased P wave amplitude
- U wave (best seen in mid-precordial leads). Typically the U wave will exceed the T wave amplitude when serum K is below 3 mEq/L
- T-W wave fusion to create giant U waves
- Ventricular tachycardia, ventricular fibrillation, and AV block are rare
Here’s our patient’s EKG at the time of arrival:
D. More Data
Additional serum and urine studies (after fluid administration):
| VBG | 7.61 / 56 / 7.1 | |
| Lactate | > 15 | mmol/L |
| Serum Osmolality | 263 | mosm/kg |
| Urine Osmolality | 211 | msom/kg |
| Urine Na | 37 | mEq/L |
| Urine K | 36 | mEq/L |
| Urine Cl | < 20 | mEq/L |
| Urine Urea | 150 | mg/dL |
| Urine Cr | 71.4 | mg/dL |
FeNa 1.8% (Fe = fractional excretion)
FeUrea 19.6%
FeK 9.4%
Urine K/Cr ratio (mmol/mmol) 5.7
Learn more about FeK and Urine K/Cr ratio from our previous post!
So..what are our patient’s active problems?
- Metabolic alkalosis
- Symptomatic hypokalemia (EKG changes)
- Hyponatremia
- Acute Kidney Injury
- Lactic acidosis
E. More on Metabolic Alkalosis
How does vomiting lead to metabolic alkalosis?
- Increase in unbuffered HCO3 due to loss of H+ in gastric fluid
- This is the normal situation where stomach acid generation creates bicarbonate which is later neutralized in the duodenum
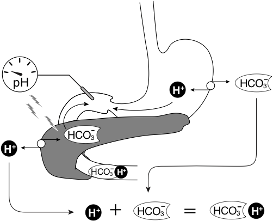
- When patient’s have vomiting, bicarbonate generated in the stomach is not neutralized and causes metabolic alkalosis.
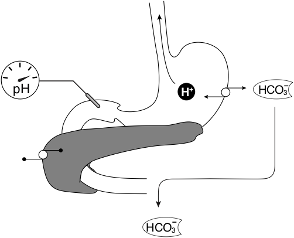
What is the mechanism for hypokalemia in this patient?
- NOT through GI losses! Gastric secretions are about half and half NaCl and HCl with only 5-20 mEq/L of potassium, so even if a patient has an NGT draining 2L of fluid daily, the loss of potassium would be no more than 40 mEq. Patients with a serum potassium of 2.4 probably have lost closer to 200-400 mEq of potassium…
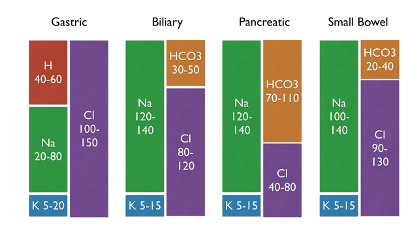
- Potassium is lost through the urine
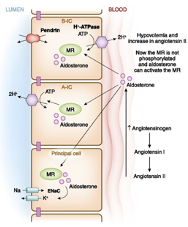
- Secondary hyperaldosteronism leads to activation of ENaC and Na/K ATPase allows potassium efflux from the principal cells Aldosterone also increases the activity of Pendrin and H/K ATPase.
- Filtered bicarbonate from metabolic alkalosis overwhelms the resorptive capacity of bicarbonate in the proximal tubule. Because the transport maximum (Tm) of bicarbonate is normally around 28, sodium bicarbonate is delivered distally. The increased distal delivery of sodium and bicarbonate both act to increase potassium excretion.
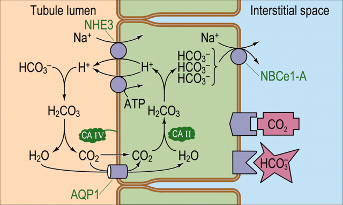
- Hypochloremia can result in urinary potassium wasting through voltage gated potassium channels.
What is the relationship between alkalosis and hypokalemia?
- It is cyclical.
- An initiating event of increased serum bicarbonate concentration has to occur, typically through one of the following four mechanisms.
- GI H+ loss (examples include congenital Cl- wasting diarrhea, laxatives, and vomiting)
- Renal H+ loss (examples include Pendred syndrome, Bartter, Gitelmans, diuretic use, mineralocorticoid excess, and hypercalcemia)
- Intracellular H+ shift (examples include hypokalemia)
- Alkali administration (examples include infusion of sodium bicarbonate, citrate, or milk alkali syndrome)
- Then something needs to maintain the alkalosis by preventing the excretion of excess bicarbonate through one of the following five mechanisms.
- Low estimated arterial blood volume leading to an increase in sodium bicarbonate reabsorption in the proximal tubule
- Hypokalemia which activates H/K ATPase and moves H intracellularly
- Hypochloremia limiting bicarbonate excretion
- Acute kidney injury leading to bicarbonate excretion
- Hyperaldosteronism
F. Breaking the Cycle
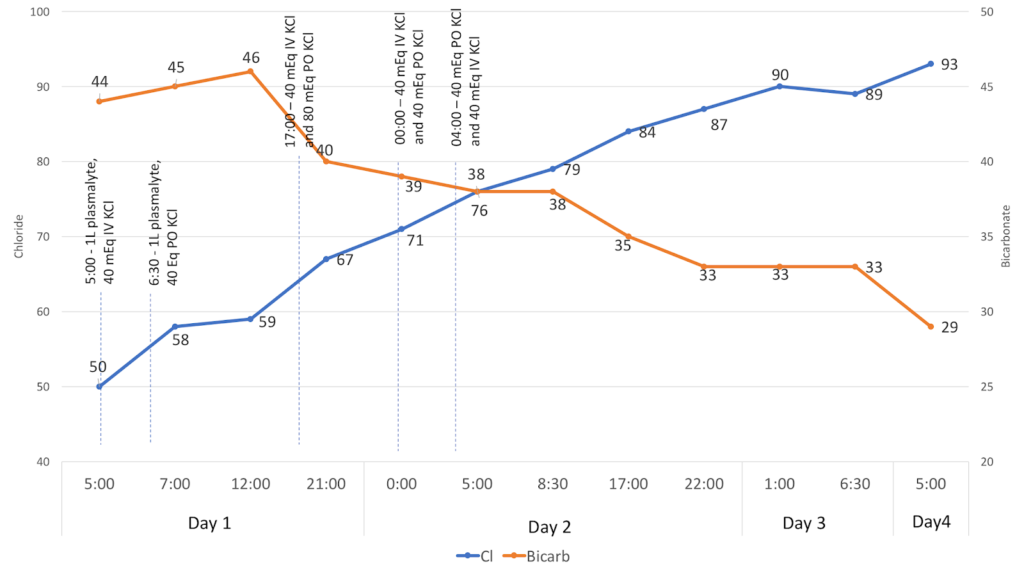
This patient had multiple initiating and maintenance factors, making it a difficult cycle to break. First thing is to fix the life-threatening hypokalemia with potassium supplementation. Second thing is to ameliorate the alkalosis and expand volume with a Cl salt infusion. There are different types of chloride infusions. Obviously sodium chloride (NaCl), potassium chloride (KCl), and do not forget hydrochloric acid (HCl).
Severe metabolic alkalosis can lead to seizures, altered mental status, myocardial hypoperfusion, and arteriolar constriction, although some believe this is due more to the low Cl rather than the alkalosis. Most would say the goal immediately is to lower the pH to less than 7.55 and lower the serum bicarb to less than 40-50 mEq/L.
HCl is not really used anymore because it is difficult to store in the pharmacy and it is pretty caustic. There are similar solutions such as ammonium chloride, which becomes urea and HCl, and arginine chloride. Ammonium chloride may not be the best choice in renal failure with concern for worsening uremia/encephalopathy, and arginine chloride is used in the US for diagnosing pituitary diseases but not for treatment of metabolic alkalosis. Arginine chloride can cause potassium to shift extracellularly. In our patient who is total body K depleted and hypokalemic this would not be so concerning, but in other patients, it can be severe. When arginine-Cl or HCl are used, a central line is needed because they are hyperosmolar.
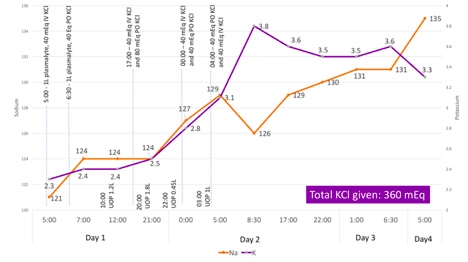
In this case, our patient’s pH was high, he was relatively asymptomatic from his alkalosis and given his severe hypokalemia, KCl is best in this case…
However, the hyponatremia was a complicating factor. He had multiple risk factors for osmotic demyelination syndrome (ODS), primarily hypokalemia and malnutrition, but the threat of hypokalemia is more immediate than the risk of ODS. Hypokalemia correction is a risk factor for overcorrection of sodium because as potassium enters the cell, it shifts hydrogen and sodium extracellularly. The hydrogen is then buffered and osmotically inactivated, thus lowering the extracellular tonicity and causing water to shift into the cell, further increasing the extracellular concentration of Na. Because of this, our correction goal for sodium was 6-8 mEq/L over 24 hours.
Here’s a cool GIF from Amy Yau on the mechanism of ODS, in the setting of hypokalemia and hyponatremia.
G. The Answer
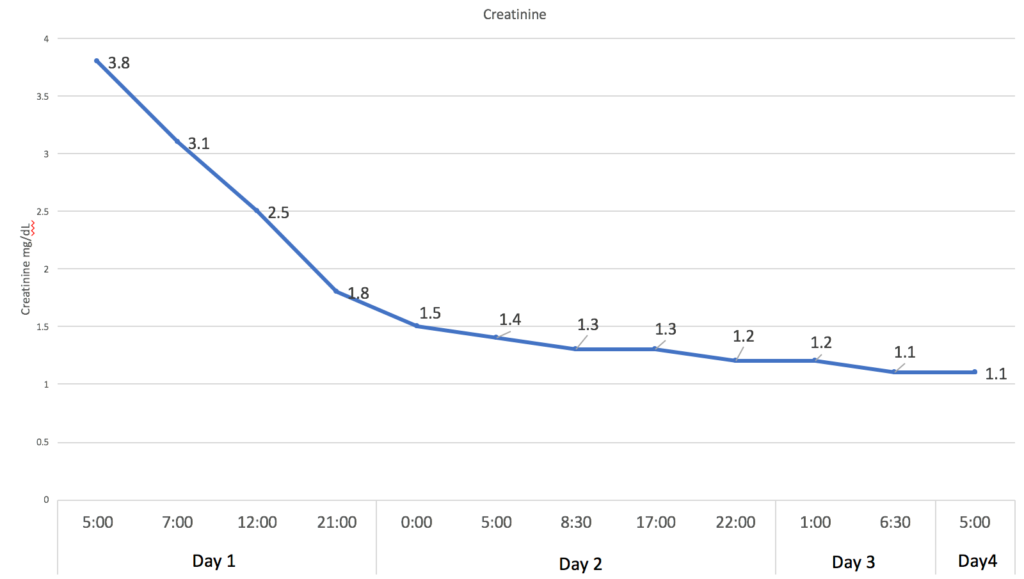
The patient was diagnosed with cannabinoid hyperemesis syndrome complicated by chloride depleted metabolic alkalosis, hypokalemia, hyponatremia, and acute kidney injury as detailed above.
The figures below illustrate how the patient corrected his electrolyte abnormalities with a total of 360 mEq of KCl and no fluid infusions, apart from the initial bolus received in the Emergency Department. His acute kidney injury improved with oral intake, and his nausea was resolved by anti-emetics.
In similar cases, patients have developed devastating ODS from aggressive treatment of potassium and sodium overcorrection.
Take Home Messages
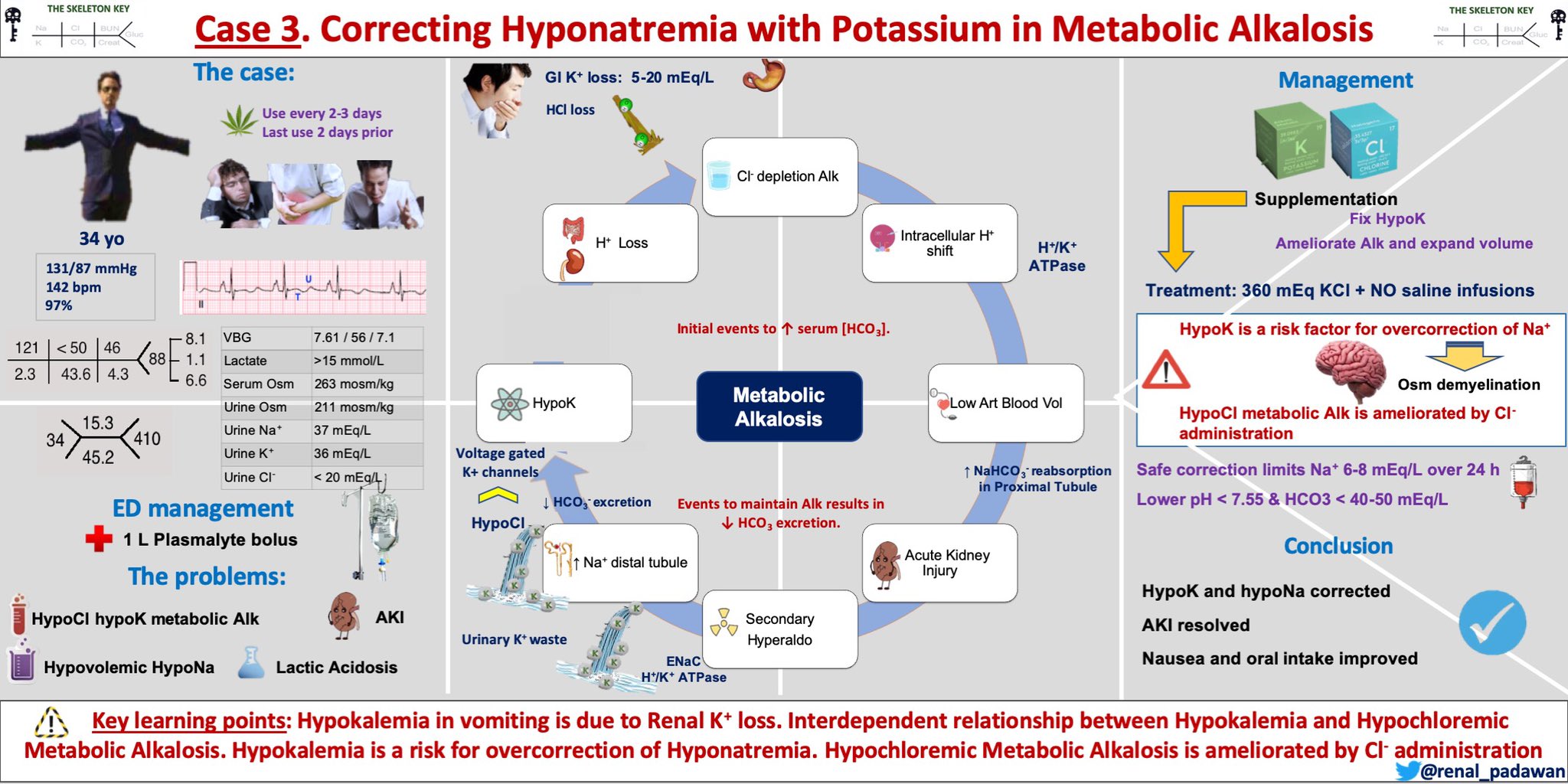
- Hypokalemia in vomiting is due to kidney potassium loss (3 mechanisms)
- There is an interdependent relationship between hypokalemia and hypochloremic metabolic alkalosis
- Hypokalemia is a risk factor for overcorrection of hyponatremia
- Hypochloremic metabolic alkalosis is ameliorated by chloride administration



Excellent teaching points. Thought provoking!
Excellent case 😍
Excellent case
thanks for pointing this out. Will fix this
images are missing!!!! please correct! interesting case!
GIF is available on Youtube as well
https://www.youtube.com/watch?v=D-235pS5B-Q
Love it! Another good teaching point is the fact that despite the Metabolic Alkalosis, there is the presence of a high AG and Lactic Acidosis keeping that pH at 7.6. If IVF given and lactic cleared, also risk worsening Alkalemia.
Very educational.
really you are doing great job
That was a great presentation. The ODS gif didn’t work for me, but all else was great. Thank you
Excellent and thought stimulating fun
Awesome Post Amy. Why lactic acid was very high in this interesting case?
great case highlighting important aspects of electrolyte mgt. In alkalosis
nice
Very interesting 👍
Nice