Systemic lupus erythematosus (SLE) is an autoimmune disease with multisystem involvement and no cure. Among patients with SLE, roughly 50% develop lupus nephritis and are at risk of developing lupus nephritis-related end-stage kidney disease (ESKD). Current therapies for lupus nephritis broadly inhibit immune system activity, although these interventions may fail to achieve full responses, leaving patients with lupus nephritis in need of more efficacious interventions. Rituximab has been used off-label for various glomerular diseases, and several clinical trials have now explored the potential of rituximab in treatment of lupus nephritis. In this Landmark Nephrology post, we will review and discuss the use of rituximab as a therapeutic agent for lupus nephritis.
Rituximab is a human/murine chimeric monoclonal antibody, which targets the CD20 antigen on B-lymphocytes. This antigen is expressed at the early pre-B-cell stage and is lost at the time of differentiation into plasma cells. Thus, targeting the CD20 antigen brings about depletion of precursor B-cells, without affecting the plasma cell population. Rituximab achieves B-cell depletion by several distinct mechanisms including:
- Complement activation following binding to CD20
- Antibody dependent cell-mediated cytotoxicity via natural killer cells
- Caspase activation leading to B-cell apoptosis.
Thus, rituximab is being increasingly used for the management of several B cell mediated disorders. While first approved by the United States (US) – Food and Drug Administration (FDA) in 1997 for the treatment of B-cell non Hodgkin’s lymphoma, rituximab has recently gained much fervour among nephrologists for the treatment of immune-mediated glomerular disorders. Research suggests that rituximab may have an independent action on the glomerular cytoskeleton and may also act as a modulator of podocyte function, analogous to cyclosporine. This makes it a very attractive agent for the treatment of glomerulonephritis.
Among glomerular diseases, the US-FDA has approved the use of rituximab only for ANCA-associated vasculitis. However, it has several off-label indications, such as in the induction and maintenance protocols of other glomerular diseases including lupus nephritis, membranous nephropathy, minimal change disease and focal segmental glomerulosclerosis (FSGS). It also has a role in kidney transplantation in desensitization protocols for HLA and ABO incompatible recipients, as well as in management of rejection episodes.
The risk of developing lupus nephritis-related ESKD at five, ten, and fifteen years is 11, 17, and 22% respectively (CI 10–12%, 16–18%, 20–23%). Current KDIGO and EULAR/ERA-EDTA guidelines recommend immunosuppression including corticosteroids, cyclophosphamide or mycophenolate mofetil (MMF) as first-line agents for remission induction in lupus nephritis. These guidelines define a complete kidney response as a return of serum creatinine (SCr) to previous level, with a decline in the urine protein creatinine ratio (uPCR) to <500 mg/g (<50 mg/mmol). A partial response is an improvement of SCr, but not to standardized normal, with at least a 50% decline in the uPCR. However, with current induction regimens, <60% of class III to V patients with lupus nephritis achieve a complete response. Thus, in spite of being a major contributor to kidney related morbidity, lupus nephritis is still in need of more effective therapeutic interventions.
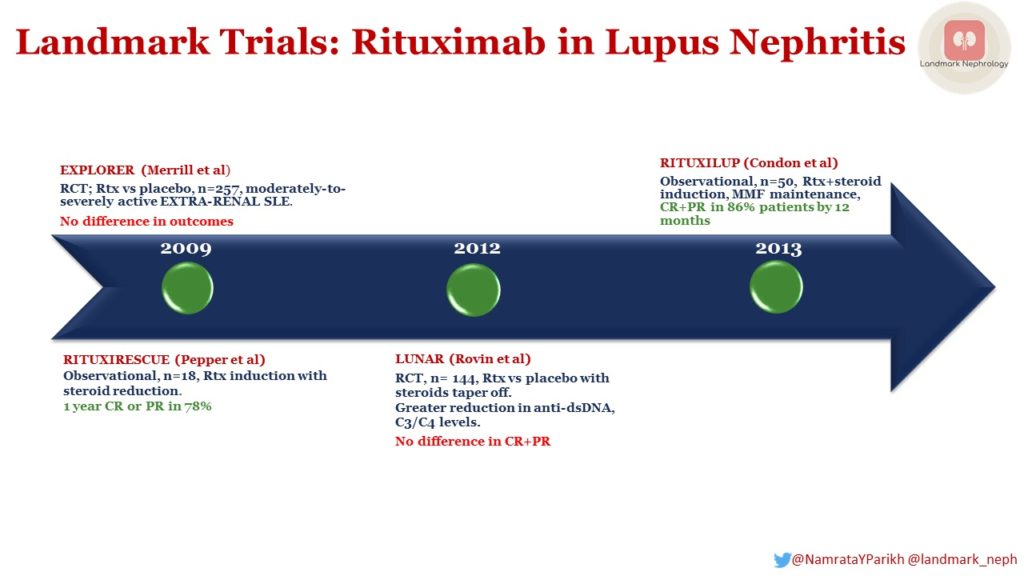
It was with the aim of filling this need, that rituximab was investigated as a possible agent for bringing about remission in systemic lupus erythematosus (SLE) in general, and lupus nephritis in particular. However, one of the first major trials in this direction was the EXPLORER (2009) RCT, involving 257 SLE patients WITHOUT lupus nephritis. Inclusion criteria included British Isles Lupus Assessment Group (BILAG) A score ≥1 or BILAG B scores ≥ 2 (BILAG divides parameters into 10 organ domains such as neurological, cardiovascular, renal etc and then scores them), despite background immunosuppression of azathioprine, methotrexate or MMF (continued during the trial). Patients were randomized to receive 1 gram rituximab or placebo on days 1, 15, 168 and 182. Cyclophosphamide was not part of treatment protocol. Patients received additional daily oral prednisone (0.5 mg/kg, 0.75 mg/kg, or 1.0 mg/kg), based on both the BILAG score and the current amount of steroids at the time of entry. This trial did not demonstrate any advantage of rituximab over placebo in either primary (proportion of patients achieving a major or partial clinical response measured by the BILAG index at 52 weeks) or secondary endpoints (time-adjusted area under the curve of BILAG disease activity, improvement in BILAG disease activity, time to flare, quality of life, and proportion taking <10 mg prednisone/day). Thus, EXPLORER failed to show any real advantage of rituximab in the treatment of SLE patients who did not have lupus nephritis.
But what about patients who did have lupus nephritis? Several studies were, in fact, carried out to evaluate the efficacy of rituximab in SLE patients WITH lupus nephritis.
Below, we review the following trials:
- RITUXIRESCUE (2009)
- LUNAR (2012)
- RITUXILUP (2013)
RITUXIRESCUE
RITUXIRESCUE (2009) was a single center, open label, prospective, observational study, involving 18 patients with class III, IV or V lupus nephritis, who were already receiving steroids for SLE. Rituximab was given at a dose of 1 g on days 1 and 15 with or without 500 mg of methylprednisolone. Cyclophosphamide was not part of treatment protocol. MMF was used for maintenance therapy. Steroid withdrawal/reduction was guided by clinical response and the presence of extra-renal manifestations. Fourteen of eighteen (78%) patients achieved complete or partial remission with a sustained response in twelve patients (67%) at 1 year. The complication rate was low with no severe infections. Following treatment with rituximab, 6 patients stopped prednisolone, 6 patients reduced their maintenance dose and 6 patients remained on the same dose (maximum 10 mg).These results indicated the possible efficacy of a rituximab and MMF-based regime with its potential to reduce the steroid requirement.
LUNAR
Next came a placebo controlled randomized controlled trial – LUNAR (2012) in which 144 class III or IV lupus nephritis patients were randomized to receive 1 g rituximab vs placebo on days 1, 15, 168 and 182. All patients also receive MMF – initiated at 1.5 g/day in 3 divided doses, which was increased to 3 g/day by week 4 as tolerated, and continued up to at least week 52. Two doses of methylprednisolone 1,000 mg were given within 3 days of therapy. Oral prednisone 0.75 mg/kg/day (maximum 60 mg) was given until day 16 and tapered to ≤10 mg/day by week 16. Other immunosuppressive agents were not permitted. Although use of rituximab achieved greater reductions in anti-dsDNA and C3/C4 levels, there was no significant improvement in clinical outcomes, namely complete responses (CR) or partial responses (PR).
RITUXILUP
However, hope for rituximab again emerged with RITUXILUP (2013), in which 50 class III, IV or V patients were enrolled in a prospective observational study to receive 1 g rituximab and methylprednisolone 500 mg on days 1 and 15. All patients received MMF, initially 500 mg twice a day, titrated (max 1.5 g twice a day) to 12-h trough mycophenolic acid (MPA) levels of 1.2–2.4 mg/L. Notably oral steroids were not used. By 52 weeks, CR and PR had been achieved in 52% (n=26) and 34% (n=17) respectively. It was concluded that rituximab may have a possible role as a steroid sparing agent in the treatment of lupus nephritis.
Rituximab in severe/relapsing/refractory lupus nephritis
Of the three studies mentioned above, only LUNAR used rituximab as an induction agent to achieve remission in lupus nephritis. RITUXIRESCUE and RITUXILUP primarily studies rituximab as a steroid-sparing agent. In addition, rituximab has been studied in the treatment of severe, relapsing or refractory lupus nephritis.
Melander et al achieved a complete remission rate (CRR) of 60% in 20 patients (retrospective study) with severe lupus nephritis.
Catapano et al were able to achieve a CRR of 91% in 11 cases (retrospective study) of refractory/relapsing lupus nephritis.
Other researchers (pooled cohorts) have been able to achieve CRRs between 30 – 90% with rituximab in refractory Lupus Nephritis.
Thus, rituximab was not useful as an induction agent in SLE with (LUNAR) or without lupus nephritis (EXPLORER). However, it does carry potential as a means to avoid steroid related adverse events (RITUXIRESCUE and RITUXILUP). It may also have a role in relapsing or refractory lupus nephritis.
To conclude, Lupus Nephritis is a serious, life threatening complication of SLE. Current approaches to treatment are associated with significant adverse effects. Rituximab, in this scenario, may have a role in avoiding the potential toxicity associated with standard immunosuppressive agents as well as in the management of patients who are refractory to or who relapse with standard agents.
Post by: Namrata Parikh


