Justine Abais-Battad, PhD
Instructor, Medical College of Georgia at Augusta University
Over the last decade we have witnessed surging scientific and public interest in the gut microbiota and its relationship to our overall health. German philosopher Ludwig Feuerbach said nearly two centuries ago,
“You are what you eat”
With recent evidence, it is becoming increasingly apparent that the gut microbiota is crucial in shaping the biochemical profile of the diet. The question though is, how? The answer lies in the complex interaction between gut microbes and what we eat. Gut-derived metabolites are key mediators in host-microbiota crosstalk by inducing cellular signaling that is either beneficial or deleterious to the host. Deleterious microbial metabolites, if persistent and chronic, can contribute to end-organ damage and ultimately diseases like hypertension, obesity, or chronic kidney disease (CKD).
The gut microbiota consists of all the microorganisms that colonize the gastrointestinal tract, including bacteria, fungi, viruses, archaea and protozoa. Together, these components function in synchrony to carry out a number of physiological and homeostatic functions:
- dietary fermentation and metabolism
- host immunity and defenses
- maintenance of gut barrier integrity
One notable way that these critical functions are modulated is via a diverse reservoir of gut microbial metabolites that are produced in response to exogenous dietary factors. Depending on the composition of the diet, the gut microbiota can regulate its systemic effects via altered production of metabolites like short-chain fatty acids (SCFAs), bile acids, and uremic toxins.
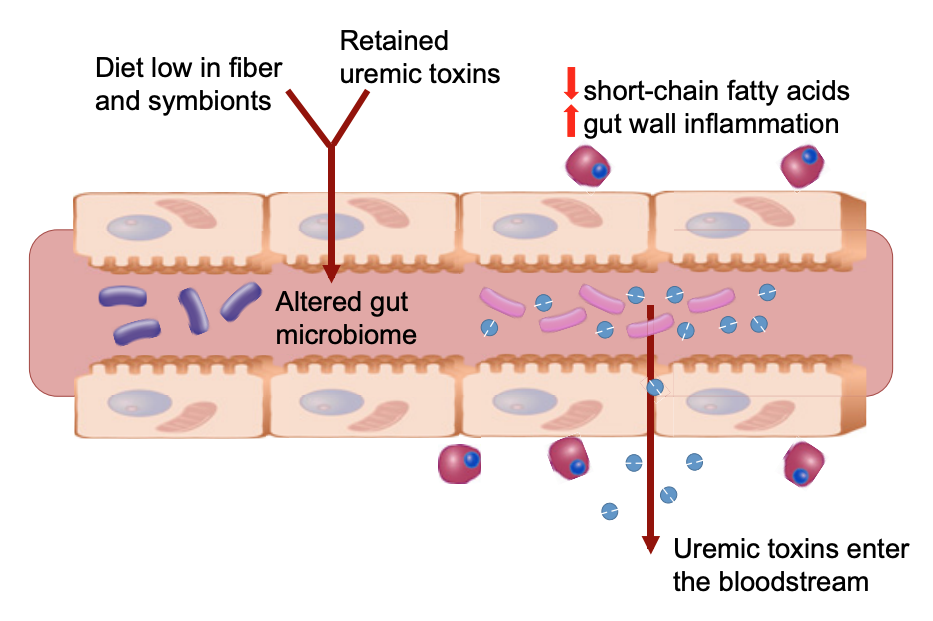
In the field of CKD, gut metabolites that affect the progression of kidney disease have recently been identified. Pioneered by Pluznick et al, gut-derived SCFAs (the beneficial byproducts of gut microbiota fermentation of dietary fiber) have been associated with improved kidney function and blood pressure-lowering effects in animal models. More specifically, treatment with the SCFA acetate resulted in improved serum creatinine and urea levels, and reduced tubular epithelial cell necrosis and inflammation in mice subjected to ischemia-reperfusion injury. Moreover, the SCFA propionate caused a dose-dependent drop in blood pressure in wild-type mice when given as an intravenous acute infusion. In people with CKD, the gut microbiome composition is characterized by a decrease in families of bacteria that produce SCFAs, like Lactobacillaceae and Prevotellaceae. Opposing the benefits of SCFAs, gut microbes also produce putative uremic toxins such as indoxyl sulfate, p-cresyl sulfate, and trimethylamine-N-oxide (TMAO). These have been associated with a number of pathologies related to cardiovascular and kidney disease by promoting oxidative stress, inflammation, and endothelial dysfunction. In people with CKD, there was also an observed expansion of bacterial families that express enzymes that contribute to the generation of these putative uremic toxins. These metabolites were able to circulate systemically due to impairments in intestinal barrier integrity observed in this group of patients with CKD (Figure 1). In combination with compromised kidney function and diminished clearance, uremic toxins are retained in the blood, which may contribute to the cycle leading to kidney function decline. In fact, plasma levels of TMAO are elevated in patients with CKD and were predictive of 5-year mortality risk. The evidence thus far implies that these gut-derived metabolites are involved in chronic disease, and there remains tremendous impetus for understanding what regulates the production of these systemic factors.
Gut microbiota research thus far, including studies of CKD, has been dominated by phenotyping the genomic profile of gut bacteria. The current dogma in microbiota research is that the diet (or drug/treatment/manipulation) shifts the relative abundances of gut bacteria, and that the change in bacterial diversity is what results in altered metabolite production. The majority of literature on the role of gut microbiota in health and disease focuses on bacterial diversity and richness as an index for ‘gut health’. However, a landmark paper by Lobel et al pushed the field to think in a drastically different perspective. In an adenine-induced dietary mouse model of CKD, the authors used a high sulfur-containing amino acid diet to induce S-sulfhydration of microbial enzymes. This diet increased the production of sulfide by gut bacteria, which led to the posttranslational modification and inhibition of the microbial-specific enzyme tryptophanase. This change in tryptophanase activity reduced uremic toxin production (indole and indoxyl sulfate), reduced serum creatinine, and improved kidney histopathology. The authors demonstrated that changes to gut microbial enzymes that regulate metabolite production, rather than altered microbial composition, contributed to the slowed progression of CKD in an adenine-diet induced mouse model. While there is more to learn regarding gut microbiome and disease, we now know that shifts in gut bacteria abundance are not the only way that the gut microbiota regulates its function and impacts host health.
The study by Lobel et al highlights the new concept that bacterial diversity alone may not be enough to get an accurate depiction of either gut or host health. This concept has significant implications not only for the treatment of CKD, but for a wide array of other chronic diseases as well. Within the next decade, we may observe a shift in the focus of studies away from the taxonomic definition of present bacterial species and more towards the understanding of the functional and metabolic processes of the gut microbiota that affect host physiology. While this new approach may prove experimentally complicated, Lobel et al provide an example for the feasibility of such studies. They have demonstrated that targeting bacterial enzymes via posttranslational modifications alters microbial function and the progression of CKD, and although there is a long way to go in the application of this data to humans, they have unveiled an entirely new class of potentially therapeutic targets.
Reviewed by: Elinor Mannon, Kelly Hyndman, PhD, Matthew Sparks, MD, FASN, FAHA
If you enjoyed reading this post and would like to learn more about dietary factors that may contribute to chronic kidney disease, consider checking out these resources on RFN:
Oxalate nephropathy: innocent bystander or primary driver of CKD


